Atoms ions and isotopes worksheet answers – Embark on an enlightening journey into the realm of atoms, ions, and isotopes, where the very building blocks of matter unfold their captivating secrets. This meticulously crafted worksheet, accompanied by comprehensive answers, empowers you to delve into the fundamental principles that govern the behavior and significance of these atomic entities.
Prepare to unravel the intricate tapestry of atomic structure, explore the intriguing differences between atoms and ions, and unravel the profound implications of isotopic variations. With each question and its corresponding answer, you will gain a deeper understanding of the properties, applications, and potential implications of these fundamental particles.
Basic Concepts of Atoms, Ions, and Isotopes
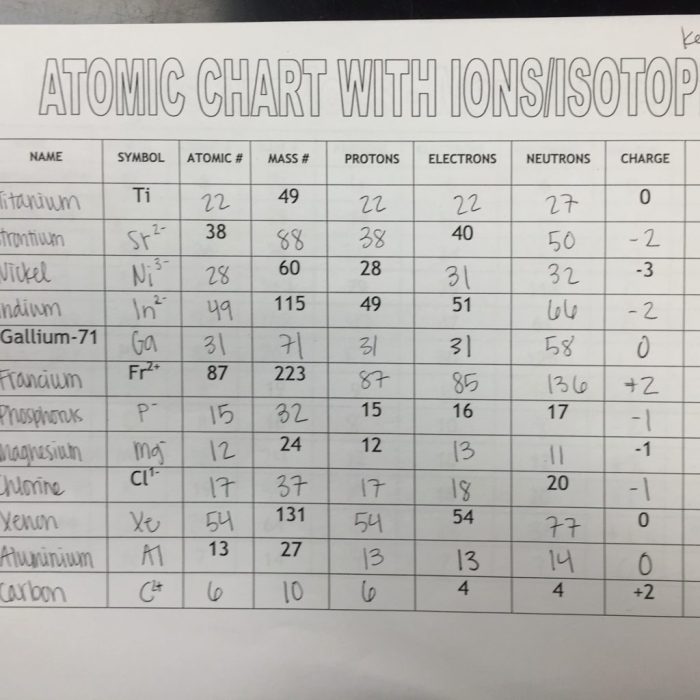
Atoms are the fundamental building blocks of matter, composed of a nucleus surrounded by electrons. The nucleus contains protons, which carry a positive charge, and neutrons, which have no charge. Electrons carry a negative charge and orbit the nucleus in specific energy levels.
Ions are atoms that have gained or lost electrons, resulting in a net electrical charge. Cations are positively charged ions that have lost electrons, while anions are negatively charged ions that have gained electrons.
Isotopes are atoms of the same element that have different numbers of neutrons. Isotopes have the same atomic number (number of protons), but different mass numbers (total number of protons and neutrons).
Properties of Atoms, Ions, and Isotopes
The atomic number of an atom is equal to the number of protons in its nucleus and determines the element’s identity. The mass number is equal to the total number of protons and neutrons in the nucleus.
The number of electrons in an atom is equal to the number of protons, resulting in a neutral overall charge. Ions have a net electrical charge due to an imbalance between the number of protons and electrons.
The stability and reactivity of ions depend on their charge and size. Smaller ions with a higher charge are generally more reactive than larger ions with a lower charge.
Isotopes and their Applications: Atoms Ions And Isotopes Worksheet Answers
Isotopes have various practical applications in different fields:
- Medicine:Isotopes are used in medical imaging techniques, such as X-rays and PET scans, to diagnose and treat diseases.
- Industry:Isotopes are employed in industrial processes, such as food irradiation and oil exploration, to improve product quality and safety.
- Scientific research:Isotopes are valuable tools for dating ancient artifacts, studying geological processes, and understanding the origin and evolution of the universe.
While isotopes offer significant benefits, it’s crucial to consider potential risks associated with their use, such as radiation exposure and environmental contamination.
Common Queries
What is the fundamental difference between an atom and an ion?
An atom is a neutral entity, possessing an equal number of protons and electrons, while an ion carries an electrical charge due to an imbalance between protons and electrons.
How do isotopes contribute to the diversity of elements?
Isotopes of the same element share the same atomic number but differ in their neutron count, resulting in variations in atomic mass and certain properties.
What are some practical applications of isotopes in medicine?
Isotopes are employed in medical imaging techniques, such as PET scans, to visualize and diagnose various physiological processes and diseases.

